closed
Marzia Caproni, Azienda Sanitaria di Firenze – Medicina Interna, Florence, Italy
3-years Project
Dermatitis Herpetiformis
Area: Clinics
- Grant: FC 017/2014
- Title: The development of dermatitis herpetiformis (DH) in celiac patients: definition of the specific T cell response against the main exogenous- and auto-antigens involved.
- Topic: Clinics
- Duration: Triennial Project
- Principal Investigator: Marzia Caproni, Direttore SOS Malattie Rare Dermatologiche, U.O. Dermatologia Ospedale Piero Palagi, USL Toscana Centro, Centro di Riferimento Regionale per la Dermatite Erpetiforme, Coordinatore Regionale per le Malattie Rare Dermatologiche, European Reference Network (ERN) – Skin Member, Florence, Italy
Publications originated from the Project:
- Bonciani D, Quintarelli L, Del Bianco E, Bianchi B, Caproni M. Serum levels and tissue expression of interleukin-31 in dermatitis herpetiformis and bullous pemphigoid. J Dermatol Sci. 2017 Aug;87(2):210-212. doi: 10.1016/j.jdermsci.2017.04.008. Epub 2017 Apr 22. PMID: 28465041. https://pubmed.ncbi.nlm.nih.gov/28465041/
- Antiga E, Maglie R, Quintarelli L, Verdelli A, Bonciani D, Bonciolini V, Caproni M. Dermatitis Herpetiformis: Novel Perspectives. Front Immunol. 2019 Jun 11;10:1290. doi: 10.3389/fimmu.2019.01290. PMID: 31244841; PMCID: PMC6579917. https://pubmed.ncbi.nlm.nih.gov/31244841/
- Caproni M, Capone M, Rossi MC, Santarlasci V, Maggi L, Mazzoni A, Rossettini B, Renzi D, Quintarelli L, Bianchi B, Ninci A, Lami G, Calabrò A, Cosmi L, Annunziato F, Liotta F. T Cell Response Toward Tissue-and Epidermal-Transglutaminases in Coeliac Disease Patients Developing Dermatitis Herpetiformis. Front Immunol. 2021 Apr 20;12:645143. doi: 10.3389/fimmu.2021.645143. PMID: 33959126; PMCID: PMC8093623. https://pubmed.ncbi.nlm.nih.gov/33959126/
Project rationale and aims
Dermatitis herpetiformis (DH) is considered the specific cutaneous manifestation of celiac disease (CD).
The reason why only few coeliac patients develop the cutaneous manifestation typical of DH, is still unknown. Epidermal transglutaminase (TG3) has been described as the main autoantigen of humoral immunity in DH but the mechanisms leading to this autoimmune response remain obscure. The present study is aimed to characterize T cells from skin, gut and peripheral blood (PB) of active DH and CD patients without skin involvement. In addition, in this study we evaluated and monitored the impact of the gluten-free diet (GFD) on circulating T lymphocytes’ phenotype and investigated antigen specific T cell response toward epidermal (TG3) and tissue transglutaminase (TG2).
Finally, we evaluated the antigen specific T cell response toward the two isoforms of transglutaminase (TG2 and TG3) in DH patients compared to the CD patients’ one.
Specifically, at first, we aimed to assess the T helper equipment in patients suffering with DH both by evaluating the production of the cytokines related to the main T helper cell subpopulations (Th1, Th2, Th17), and by the assessment of T cell antigen specificity. Subsequently we aimed to evaluate the antigen specificity of T cells considering T cell proliferative response toward TG2 and TG3, the two main autoantigens involved in CD and in DH, respectively.
Research plan and results obtained
The comparison between the results obtained from the skin, gut and PB in DH patients vs. non DH CD patients showed a significant increase in TNFα-producing CD4+ T cells in the skin of DH patients, and a nearly significant (p=0.054) increase of skin derived CD4 + T cells producing IL-4 in DH patients. The frequency of IL-17 producing CD4+ T cells was higher in the skin of DH patients when compared to PB. No differences raised between the frequency of cytokine producing TCLs deriving from PB of healthy donors. These results are in agreement with published data describing a key role of neutrophils and eosinophils in the induction of skin damage in DH.
Moreover, we found that either in DH or CD, gut-derived T cell lines were enriched in IFN-γ producing CD4+ T cells compared to peripheral blood (PB) (Figure 1).
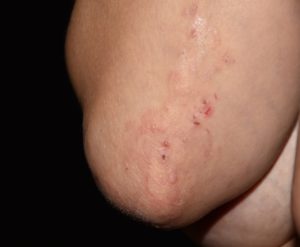
Figure 1: TNFα-producing cells are enriched in skin samples from DH patients. (A) Intracellular cytokines production in in vitro expanded CD4+ T cells from PB (black columns), skin (grey columns) and gut (white columns): frequencies of cytokines producing of CD4+CD3+-gated cells were assessed in 10 DH patients, 6 CD patients and 4 healthy donors. Columns represent means ( ± SE). Bars * indicate p ≤ 0.05; bars ** indicate p ≤ 0.01. (B) Frequencies of intracellular cytokine producing CD4+ T cells in fresh samples from PB (black columns), skin (grey columns) and gut (white columns) of 5 DH patients. Columns represent means ( ± SE). Bars * indicate p ≤ 0.05. (C) Representative flow cytometric analysis obtained in one out of five subjects with DH and in only one healthy specimen. Percentages of CD4+ T cells are shown. PB,peripheral blood.
Results on CD8+ T cells only showed that Tc1 cells were significantly increased in the gut of DH patients compared to CD ones.
Finally, the chemokine receptor expression on both CD4+ and CD8+ T cells resulted comparable among the different tissues.
Interestingly, a positive and significant correlation between the frequency of TNF-α and IL-17A-producing T-cells in the PB and the disease activity was observed in DH patients. Moreover, a significant and progressive decrease of the frequency of TNF-α-producing cells was observed after the first year of GFD, and a further decrease was appreciated after the second year; a similar trend was appreciated also for IL-17 (Figure 2).
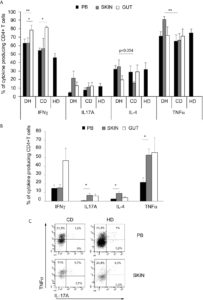
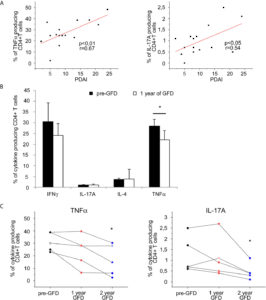
Figure 2 PB Cytokine-producing T cells frequencies correlation with disease activity score and evaluation after gluten free diet in DH patients.(A) Correlation between disease activity score PDAI and frequencies of circulating CD4+ T cells producing TNFα (left panel) and L-17A (right panel) was calculated in 15 DH patients.(B)Frequencies of intracellular cytokines producing CD4+ T cells infreshsamples from PB were evaluated in 8 DH patients before and after 1 year of gluten free diet (GFD). Columns represent means ( ± SE) of the indicated cytokine. *p ≤ 0.05.(C) Frequencies of circulating CD4+ T cells producing TNFα (left panel) and IL-17A (right panel) in fresh samples from PB were evaluated before (To) and after 1 and 2 year of gluten free diet (GFD) in 5 DH patients. ( ± SE). *p ≤ 0.05 compared to the To evaluation.
DH and CD patients’ T Cells showed a crossed proliferative response to transglutaminases; cross-reacting T cells originated from a single clone as shown by the flowcytometric analysis of the TCR Vb repertoire (Figure 3).

Figure 3 Transglutaminases-specific T cells proliferation assays. (A) Proliferation assay was performed on PB derived TCLs, induced in the presence of TG2 and TG3, from 15 DH patients (left panel) and 12 CD patients (right panel). Each TCLs was stimulated with/without both TG2 and TG3 antigens. Dots with the same shape and colour represent stimulation index (SI) of TCLs derived from the same patients. (B) Proliferation assay was performed on T cell clones (TCCs), derived from antigen specific TCLs from 1 DH patients. Each TCC was stimulated with/without both TG2 and TG3 antigens as well as streptokinase (SK) as negative unrelated control. Dots with the same colour represent SI of the same TCC.
Experimental design and methodologies
Nineteen patients with DH were enrolled in the Dermatology Department, Rare Diseases Unit of Florence from December 2014 to July 2018, while 13 patients with were enrolled in the Gastroenterology department of the University of Florence during the same period.
For each DH and CD patient, personal data, clinical findings, HLA haplotype, duodenal and skin biopsies were collected.
The phenotype of the T-cell response in the peripheral blood, gut and skin of DH and CD patients has been evaluated by means of flow cytometry analysis of the expression of membrane receptors and cytokines production as well as of the TCR Vbeta repertoire. The analysis was performed at both diagnosis and after GFD diet. The antigen-specific T-cell response was evaluated by ex-vivo stimulation of cross-reactive T-cell clones with the antigen of interest (either Tg2 or TG3) and evaluating the ability to proliferate by a thymidine incorporation assay.
Potential pitfalls and caveats
The results of our project are accepted for publication on Frontiers in Immunology. The main limitation of the study relies on the small samples size, which is consistent with the rarity of DH.
Conclusions and discussion
The present study provides new insight concerning the immunology of DH. In particular, our results demonstrate the existence of a T cell cross-reactivity between TG2 and TG3, as a basis for the T cell recognition of TG3, the crucial autoantigen in DH.
Moreover, they suggest the involvement of a Th17 skewed response at skin level during DH manifestation, with a marked proinflammatory feature, driven by TNF-α production. These data offer a new scenario into DH pathogenesis and open new possible therapeutic strategies in the field of immunotherapy, oriented to target TNF-α or IL-17A. This is particularly important in patients with severe skin exacerbation during the period of latency before the clinical response to the diet, as well as in refractory patients.
