Closed
Giovanni Monteleone University Tor Vergata, Rome, Italy
3-years Project
Celiac Disease
Area: Immunology
- Grant: FC 006/2015
- Title: Role of mTOR kinase cascade in celiac disease
- Topic: Celiac Disease, Immunology
- Duration: Triennial Project
- Principal Investigator: Prof. Giovanni Monteleone, University Tor Vergata, Rome
- Partnerships: Silvia Sedda, Vincenzo Dinallo, Irene Marafini, Alfredo Colantoni, Unità di Gastroenterologia, Università di Roma Tor Vergata
Publications originating from the Project:
- Sedda et al. mTOR sustains inflammatory response in celiac disease. Sci Rep. 2020 Jul 1;10(1):10798. doi: 10.1038/s41598-020-67889-4. https://pubmed.ncbi.nlm.nih.gov/32612145/
THE STUDY
Project rationale and aims
Celiac disease (CD) is an enteropathy triggered by the ingestion of gluten proteins in genetically predisposed individuals and characterized by excessive activation of effector immune cells and enhanced production of inflammatory cytokines. In particular, gluten peptides stimulate both epithelial cells and innate immune cells to secrete interleukin (IL)-15, which in turn activates both CD8+ and natural killer + cells thereby contributing to the gluten-driven epithelial damage. Moreover, gluten exposure facilitates differentiation of CD4+ T-cells in T helper (Th)-type 1 cells. These gluten-specific Th1 lymphocytes synthesize interleukin (IL)-21 and interferon (IFN)-γ, two cytokines that activate several intracellular pathways and contribute to establish positive feedback loops amplifying the ongoing mucosal immune response. However, little is known about mechanisms which sustain and perpetuate the detrimental immune response in CD. The mammalian target of Rapamycin (mTOR) pathway acts as an important integrator of nutrient-sensing pathways, which control and coordinate the metabolism of the cell according to its need to proliferate or functionally differentiate. The hyper-activation of mTOR occurs in many immune-inflammatory diseases. The aim of this project was to investigate the role of mTOR kinase cascade in the CD-related mucosal inflammation.
Research plan and results obtained
By Western blotting and immunofluorescence, we showed a marked increase of active mTOR in duodenal samples of active CD patients as compared to normal controls. Notably, an intense and diffuse staining for active mTOR was seen in the epithelial compartment of active CD patients, while mTOR was barely detectable in lamina propria mononuclear cells (Figure 1).
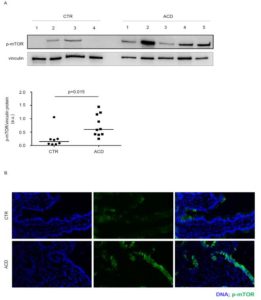
Figure 1. Phosphorylated (p)/active form of the mammalian target of Rapamycin (mTOR) is up-regulated in active celiac disease patients. A) Western blots showing p-mTOR and vinculin (used as a loading control) expression in duodenal biopsy samples taken from 4 normal controls (CTR) and 5 active celiac disease (ACD) patients. The blots are representative of 2 separate experiments in which total proteins extracted from duodenal biopsy samples of 8 CTR and 10 ACD were analyzed. The panel shows quantitative analysis of p-mTOR/vinculin ratio in each sample as measured by densitometry scanning of all Western blots; horizontal bar indicates median value. Values are expressed in arbitrary units (a.u.). B) Representative images of immunofluorescence staining of duodenal sections of CTR and ACD patients for p-mTOR (green staining). DNA is stained with DAPI (blue).
To determine which complex is activated in CD mucosa, we initially evaluated expression of p-Raptor, a component of the mTORC1 complex. Immunoreactive bands for p-Raptor were more evident in ACD as compared to controls (Figure 2A). Next, we analysed expression of p-4EBP, an indicator of mTORC1 activation. Evaluation of p-4EBP confirmed marked activation of mTOR in active CD as compared to controls (Figure 2B). We have also evaluated p-Rictor, a component of mTORC2 complex, in the same duodenal samples used to assess p-4EBP. p-Rictor expression did not differ between active CD and controls (Figure 2C). Altogether, these data indicate that active CD-related inflammation is marked by activation of mTORC1 and not mTORC2. To evaluate whether changes in mTOR kinase cascade are simply due to the ongoing duodenal inflammation, we analysed p-4EBP in duodenal samples of 2 patients with Whipple disease (WD) and 2 patients with common variable immunodeficiency (CVID). p-4EBP was detectable in all these samples with no apparent difference between CVID, WD, and controls (Figure 2D).
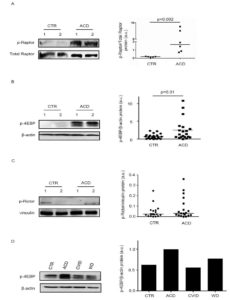
Figure 2. mTOR complex 1 (mTORC1) is activated in active celiac disease. A). Western blots showing phosphorylated (p) and total forms of Raptor in duodenal biopsy samples taken from 2 normal controls (CTR) and 2 active celiac disease (ACD) patients. The blots are representative of 2 separate experiments in which total proteins extracted from duodenal biopsy samples of 5 CTR and 6 ACD patients were analyzed. The right panel shows quantitative analysis of p-Raptor/total Raptor ratio in each sample as measured by densitometry scanning of all Western blots; horizontal bar indicates median value. Values are expressed in arbitrary units (a.u.). B) Western blots showing phosphorylated (p)-4E-binding protein (4EBP) and β-actin (used as a loading control) expression in duodenal biopsy samples taken from 2 normal controls (CTR) and 2 active celiac disease (ACD) patients. The blots are representative of 6 separate experiments in which total proteins extracted from duodenal biopsy samples of 23 CTR and 20 ACD were analyzed. The right panel shows quantitative analysis of p-4EBP/β-actin ratio in each sample as measured by densitometry scanning of all Western blots; horizontal bar indicates median value. Values are expressed in arbitrary units (a.u.). C) Western blots showing phosphorylated (p)-Rapamycin-insensitive companion of mammalian target of rapamycin (Rictor) and vinculin (used as a loading control) expression in duodenal biopsy samples taken from 2 CTR and 2 ACD patients. The blots are representative of 2 separate experiments in which total proteins extracted from duodenal biopsies of 19 CTR and 19 ACD were analyzed. The right panel shows quantitative analysis of p-Rictor/vinculin ratio in each sample as measured by densitometry scanning of all Western blots; horizontal bar indicates median value. Values are expressed in arbitrary units (a.u.). D) Western blots showing p-4EBP and β-actin (used as a loading control) expression in duodenal biopsy samples taken from 1 CTR, 1 patient with ACD, 1 patient with Whipple disease (WD) and 1 patient with common variable immunodeficiency (CVID). The right panel shows quantitative analysis of p-4EBP/β-actin ratio as measured by densitometry scanning of all Western blots.
Stimulation of inactive celiac disease (ICD) samples with pepsin-trypsin-digested (PT)-gliadin for 24 hours consistently enhanced p-4EBP expression, suggesting that mTOR pathway is activated by a gliadin-driven inflammatory response. CD-associated inflammation is marked by activation of gluten-specific T cell clones, which produce IFN-γ and IL-21. Therefore, we next investigated the involvement of these 2 cytokines in the activation of mTOR. Both IFN-γ and IL-21 enhanced p-4EBP expression. Many biological functions of IFN-γ and IL-21 are mediated by the Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway. In our experiments, treatment of biopsy samples with AG490 (a JAK/STAT inhibitor) reduced IFN-γ/IL-21-induced p-4EBP expression. Finally, we assessed whether mTOR regulates IL-15 synthesis. Rapamycin, an inhibitor of mTORC1, markedly inhibited p-4EBP expression and this associated with a reduction of IL-15 production (Figure 3).
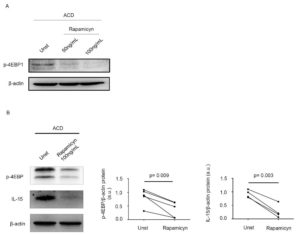
Figure 3. Inhibition of mTOR signalling reduces interleukin (IL)-15 expression in active celiac disease. A) Representative Western blot showing p-4EBP expression in duodenal biopsy samples taken from 1 patient with active celiac disease (ACD) either left untreated (Unt) or treated with 50ng/mL, 100ng/mL rapamycin for 24 hours; β-actin was used as a loading control. B) Duodenal biopsy samples taken from 4 ACD patients were either left untreated (Unt) or treated with 100ng/mL rapamycin for 24 hours and p-4EBP and IL-15 expression was assessed by Western blotting. β-actin was used as a loading control. The blots are representative of 4 separate experiments in which similar results were obtained. The right insets show quantitative analysis of p-4EBP/β-actin and IL-15/β-actin ratio as measured by densitometry scanning of all Western blots. Values are expressed in arbitrary units (a.u.).
Experimental design and methodologies
We investigated expression of active mTOR in CD patients and controls by Western blotting (WB) and immunofluorescence. To confirm activation of mTOR in CD, total proteins extracted from additional duodenal samples of patients and controls were analysed for the content of p-4EBP, p-Raptor and p-Rictor by WB. p-Raptor is a component whereas p-4EBP is a down-stream effector of mTORC1 and, therefore, their expression is indicative of mTORC1 activation. In contrast, p-Rictor is a component of mTORC2. WB was also used to evaluate expression of p-4EBP in other gastrointestinal pathologies (i.e. WD, CVID). Mucosal biopsy samples of ICD patients were placed on transwell in the absence or presence of PT; additional mucosal biopsy samples of ICD patients were placed on transwell in the presence or absence of IFN-γ or IL-21. In parallel, samples were cultured with or without cytokines in the presence or absence of the JAK/STAT inhibitor AG-490. In further experiments, mucosal biopsy samples of ACD patients were cultured in the presence or absence of rapamycin. Next the samples were used for protein extraction to assess p-4EBP expression by WB.
Potential pitfalls and caveats
Some components of the mTOR cascade were analyzed in a limited number of patients due to the difficulty to collect simultaneously many biopsy samples during endoscopy. Therefore, further work is needed to confirm the present results. It remains unclear if there is a cell-specific regulation of mTOR activation in CD as the functional studies were performed using whole mucosal samples rather than single cell types. Further longitudinal studies will be also needed to ascertain the impact of gluten-free diet on mTOR activation. Although no mTOR-positive cell was localized in the lamina propria compartment of CD patients by immunohistochemistry, more sensitive techniques (e.g. flow-cytometry) could help establish if activation of such a kinase occurs in specific immune cell types.
Conclusions and discussion
Data of the present study suggest that mTOR is constitutively activated in the human duodenum and expression of the active protein is markedly increased in ACD as compared to controls. Immunofluorescence analysis showed that epithelial cells were the only source of mTOR in both controls and patients and confirmed the more pronounced expression in ACD. mTOR functions depend on two distinct multiprotein complexes, namely mTORC1 and mTORC2. Data of the present study suggest that mTORC1 and not mTORC2 is hyper-activated in ACD mucosa. Indeed, we showed enhanced expression of p-Raptor, a component of mTORC1, and p-4EBP, a downstream effector of mTORC1 signaling, in duodenal samples of ACD as compared to normal controls while expression of p-Rictor, an indicator of mTORC2 complex activation, did not differ between ACD and controls.
The exact factors/mechanisms that activate mTOR in ACD remain to be ascertain. It is likely that the sustained activation of this kinase is not simply due to the ongoing mucosal inflammation, as we were not able to detect any change in p-4EBP in the duodenum of patients with other enteropathies (i.e. CVID and WD). At the same time, our findings support the hypothesis that mTOR can be positively regulated by gluten-driven inflammation. Indeed, ex vivo treatment of ICD samples with PT enhanced p-4EBP. We do not yet know which sequence of events is needed to induce mTOR in PT-treated biopsy samples. A possibility is that PT actives directly mTOR in line with the demonstration that intestinal epithelial cells can respond to PT by increasing production of IL-15. It is also conceivable that PT can trigger an immune response that ultimately controls mTOR activation. Support to this hypothesis comes from the demonstration that both IFN-γ and IL-21, two cytokines over-produced by gluten-specific Th1 cells in ACD, were able to enhance p-4EBP in ICD duodenal samples. The increased expression of mTORC1-related proteins in the duodenum of active CD patients and the demonstration that inhibition of such a pathway leads to a marked down-regulation of IL-15 indicate that mTOR pathway can amplify detrimental signals that ultimately promote mucosal damage.
Altogether these findings are novel and suggest that ACD inflammation is marked by activation of mTORC1 in the epithelial compartment.
