closed
Giuseppina Bologna, University “G.d’Annunzio” Chieti-Pescara, Italy
Triennial Fellowship
Celiac Disease
Area: Biology
- Grant: FC 004/2016
- Title: Characterization of Circulating Microvesicles in the peripheral blood of Celiac’s Disease Patients as a potential prognostic tool of related complications
- Duration: Triennial Project
- Principal Investigator: Giuseppina Bologna, Department of Medicine and Ageing Sciences, Center for Advanced Studies and Technology (C.A.S.T.), University “G. D’Annunzio”, Chieti-Pescara, 66100 Chieti, Italy
- Head Lab (Tutor): Professor Sebastiano Miscia, Department of Medicine and Ageing Sciences, Center for Advanced Studies and Technology (C.A.S.T.), University “G. D’Annunzio”, Chieti-Pescara, 66100 Chieti, Italy
Partnerships: Prof Matteo Neri, Department of Medicine and Ageing Sciences, University “G. D’Annunzio”, Chieti-Pescara, 66100 Chieti, Italy and Digestive Endoscopy and Gastroenterology Unit, SS Annunziata Hospital, ASL2 Abruzzo, Italy
Publications originated from the Project:
- Efthymakis K, Bologna G, Simeone P, Pierdomenico L, Catitti G, Vespa S, Milano A, De Bellis D, Laterza F, Pandolfi A, Pipino C, Sallese M, Marchisio M, Miscia S, Neri M, Lanuti P. Circulating Extracellular Vesicles Are Increased in Newly Diagnosed Celiac Disease Patients. Nutrients. 2022 Dec 23;15(1):71. doi: 10.3390/nu15010071. PMID: 36615729. https://pubmed.ncbi.nlm.nih.gov/36615729/
- Clemente E, Efthymakis K, Carletti E, Capone V, Sperduti S, Bologna G, Marchisio M, Di Nicola M, Neri M, Sallese M. An explorative study identifies miRNA signatures for the diagnosis of non-celiac wheat sensitivity. PLoS One. 2019 Dec 13;14(12):e0226478. doi: 10.1371/journal.pone.0226478. Erratum in: PLoS One. 2020 Mar 27;15(3):e0231273. PMID: 31834915; PMCID: PMC6910677. https://pubmed.ncbi.nlm.nih.gov/31834915/
- Simeone P, Celia C, Bologna G, Ercolino E, Pierdomenico L, Cilurzo F, Grande R, Diomede F, Vespa S, Canonico B, Guescini M, Stocchi V, Lotti LV, Guagnano MT, Stellin L, Papa S, Trubiani O, Marchisio M, Miscia S, Lanuti P. Diameters and Fluorescence Calibration for Extracellular Vesicle Analyses by Flow Cytometry. Int J Mol Sci. 2020 Oct 23;21(21):7885. doi: 10.3390/ijms21217885. PMID: 33114229; PMCID: PMC7660682.https://pubmed.ncbi.nlm.nih.gov/33114229/
- Marchisio M, Simeone P, Bologna G, Ercolino E, Pierdomenico L, Pieragostino D, Ventrella A, Antonini F, Del Zotto G, Vergara D, Celia C, Di Marzio L, Del Boccio P, Fontana A, Bosco D, Miscia S, Lanuti P. Flow Cytometry Analysis of Circulating Extracellular Vesicle Subtypes from Fresh Peripheral Blood Samples. Int J Mol Sci. 2020 Dec 23;22(1):48. doi: 10.3390/ijms22010048. PMID: 33374539; PMCID: PMC7793062. https://pubmed.ncbi.nlm.nih.gov/33374539/
Project rationale and aims
- Characterize and enumerate circulating extracellular vesicles (EVs) in the peripheral blood of Celiac’s Disease (CD) patients and Inflammatory Bowel Disease patients;
- Determine whether EV composition and/or absolute numbers may help to find specific diagnostic and/or prognostic tools, or biomarkers for the response to diet monitoring.
Extracellular vesicles (EVs) are hypothesized effectors of cellular cross-talk, carrying surface receptors characteristic of their origin and shuttling molecules potentially controlling immune response. Previous studies have demonstrated an increased number of circulating EVs in conditions such as insulin-resistance and atherosclerosis. In Inflammatory Bowel Disease (IBD), increased numbers of total and platelet-derived EVs have been reported, using single/multi-step centrifugation-based methods. Celiac disease (CD) is an immune-mediated inflammatory enteropathy, elicited by gluten ingestion in genetically susceptible individuals. It is frequently associated with a variety of systemic conditions both autoimmune and potentially immune-mediated in nature.
Research plan and results obtained
1) We have evaluated 22 celiac adults (mean age=40±12 years, sex ratio F/M=3:1) at diagnosis and age- and sex-matched celiac disease patients under gluten free diet (GFD) and healthy controls (HC). Mean anti-tTG levels at inclusion were 6.9±3.5 times ULN in newly diagnosed CD patients. Histology was considered positive for lesions of grade ≥ B1 according to the Corazza-Villanacci classification: partial/total atrophy ratio was 10/6. Gluten-free regimen mean duration was 2.36±0.81 years; adherence was assessed by anti-tTG negativity (0.6±0.2 times ULN) at the time of inclusion in this study.Flow cytometry analyses allowed crisp distinction of circulating EVs from exosomes, platelets and erythrocytes. Specific membrane receptor labelling (CD31, CD45, CD41a, CD326) allowed EVs subtyping, according to tissue of origin. Annexin V positivity was also assessed.
Overall, mean number of total circulating EVs was significantly higher in newly diagnosed CD patients than in controls (30283.1±6604.1 vs 14203.7±2681.3 EV/microL, p=0.026), while this was not the case for total annexin V+ EV counts (4044.8±1560.8 vs 2639.4±584.8 EV/microL, p n.s.). Celiac disease patients on GFD showed marginally higher counts of circulating EVs when compared to controls, both overall (26804±12740.5 EV/microL) and for Annexin V+ EVs (4023.5± 2212.4 EV/microL), not reaching statistical significance.Subpopulation analysis showed that EpCAM+, of epithelial origin, and CD41a+ platelet-derived EVs were significantly increased in CD compared to controls, while no differences were observed between the GFD group and HC. Notably, CD45+ annexin V+/- EVs, of leukocyte origin, showed significantly higher numbers in CD patients regardless of gluten exposure, than in HC, as was the case for CD31+ Annexin V+/-, and EpCAM+ Annexin V+.
Considering CD patients overall, presence of villous atrophy at histology (grade ≥B1 according to the Corazza-Villanacci classification) did not correlate to the total circulating EV counts (41228.7±2140.4 vs 39518.2±6958.6, p n.s.). However, EpCAM+ MVs where markedly higher in the presence of atrophy (391.83±36.4 vs 129.63±12.3, p=0.06). Furthermore, while intraepithelial lymphocyte (IEL) percentages also did not correlate to total MV counts, EpCAM+ epithelial MV counts were the only significant predictor of IEL concentrations, in a multiple linear regression model including CD31+, CD45+ and CD41a+ counts (R2 linear=0.496, p=0.022; Pearson’s r=0.676, Fig.1).
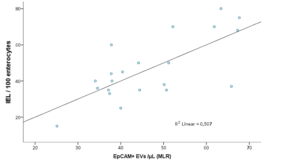
Figure 1: Correlation of IEL % and R model-predicted EpCAM+ counts in a multiple linear regression model including EpCAM+, CD31+, CD45+ and CD41a+ counts, where only EpCAM+ was a statistically significant predictor.
2) Moreover, we compared 12 IBD patients (mean age=39.9±13.8 years,F/M=1:1) with age/sex-matched HC. Disease activity was assessed by endoscopy, C-reactive protein (CRP) and fecal calprotectin levels (FC). Mean number of total EVs was significantly higher in IBD than in HC (3798±1525 vs2738±1213 EV/microL, p<0.05). Active IBD showed significantly higher EV counts compared to either IBD in remission or controls (5140±1007 vs 2704±919 and 2443±822, p<0.05). Subgroup analysis showed that EpCAM+ EVs were also significantly higher in active IBD (570±333 vs 191±219 and131±322, p<0.05). CD41+ EVs were increased in both IBD subgroups(1834±1114 and 1389±992 vs 955±569 respectively, p<0.05). Interestingly, while Annexin V+ EV counts were similar between groups, the CD45+/CD45+Annexin+ EV ratio was significantly higher in both IBD groups(2.9±0.9 and 2.2±0.7 vs 1.3±0.7, p<0.05), while the CD31+/CD31+Annexin+ ratio was increased in active IBD only (5.5±3.2 vs 2.3±0.7 and 2.4±2.2, p<0.05). FC was correlated to total EV, CD41+, EpCAM+ and CD31+ counts (R 0.722, 0.468, 0.267 and 0.249), while CRP to total Annexin V+ EV counts and the CD31+/CD31+Annexin+ ratio (R 0.425 and 0.347).
Experimental design and methodologies
1) We enrolled consecutive adult anti-tTG positive, biopsy proven CD patients, IBD patients and healthy controls (HC).Circulating EVs were assessed on whole blood samples by a no-lyse/no wash method, combined with EVs volumetric count (FACSVerse, BD), based on a novel six colour flow cytometry panel, in order to identify and enumerate both the whole EV compartment and different EVs subpopulations. In details, peripheral blood (PB) was drawn by venipuncture (21 G needle)in sodium citrate tubes and processed within 2-4 hours from collection. The first 3 ml tube was excluded from the analysis in order to minimize the impact of venipuncture-induced vascular damage that may affect EV count. For each analysis, the lipophilic cationic dye (LCD, BD Biosciences–Catalogue, #626267, Custom Kit), a Pan-EV dye recently developed in our Laboratories, was added to 195 µl of 1X binding buffer (Becton Dickinson Biosciences – BD, San Jose, CA, USA), together with a mix of reagents, as detailed in Table 1. 5 µl of PB were then added to the reagent mix and incubated at RT for 45 minutes in the dark; 500 µl of 1X binding buffer (BD Biosciences) were finally added to each tube and 1 x 106 events/sample were acquired by flow cytometry (FACSVerse, BD – three laser, eight color configuration), by setting a low threshold (200) on the channel in which the lipophilic cationic dye emits (APC-H channel). Amplifier settings for forward scatter (FSC) and side scatter (SSC) as well as for any fluorescence channel was set in logarithmic mode, and all parameters were visualized respect to the relative height (H) signal.
Table 1. List of flow cytometry specificities and reagents. | |||||
Detection | Fluorochrome | Vendor | Ab Clone | Catalog | Amount per Test |
Phalloidin | FITC | BD Biosciences | – | 626267 (custom kit) | 0.5 µl |
CD41a | PE | HIP8 | 626266 (custom kit) | 5 µl | |
CD31 | PE-Cy7 | WM59 | 5 µl | ||
CD45 | APC-H7 | BD Biosciences | 2D1 | 560178 | 2 µl |
CD326 | PerCP-Cy5.5 | BD Biosciences | EBA-1 | 347199 | 5 µl |
Annexin V | V500 | BD Biosciences | – | 561501 | 1 µl |
CD235a | BV421 | BD Biosciences | GA-R2 (HIR2) | 562938 | 5 µl |
Keys: R-phycoerythrin (PE); PE-Cyanine 7 (Cy7), Allophycocyanin-Hilite®7 (APC-H7), Peridinin-Chlorophyll-protein-Cyanine 5.5 (PerCP-Cy5.5), Brilliant Violet (BV). Becton Dickinson (BD) Biosciences (San Jose, CA, USA). | |||||
Data are expressed as mean±SD; Linear regression, Kruskal-Wallis or Mann-Whitney U tests, were used as appropriate
2) Circulating Microvesicles separation by instrumental cell sorting
The extracellular vesicles were separated (100 um nozzle) from whole samples on the basis of their positivity pattern to the LCD(lipophilic cationic dye ), their negativity to the phalloidin (which stains actin in damaged vesicle membranes) combined by their SSC-H and FSC-H features, by using a FACSAria III cell sorter (BD Biosciences). The post-sorting purity was assessed by re-analyzing instrumental-sorting purified samples, as recently recommended (Cossarizza et al. 2017). One million of purified EVs from12 healthy controls and 12 celiac adults samples were analyzed by proteomics.
Potential pitfalls and caveats
The flow cytometry protocol, used for the first time in this field, allows the analysis of freshly drown peripheral blood samples. For this reasons, this analysis must be performed within 4 h from blood collection. Frozen samples are not admitted as well. To overcome this limitation, all the analyses have been performed on fresh peripheral blood samples.
Conclusions and Discussion
All along the project, we enrolled a population of patients and control subjects that already allowed obtaining statistically significant results. IBD patients show higher numbers of circulating EVs than HC, correlating to active disease status and FC/CRP levels. Phenotypical characterization suggests that while some components of the EV compartment, such as the epithelium-derived EpCAM+, are linked to disease activity, others, like the platelet-derived CD41+, could be linked to underlying non-inflammatory immune factors and extraintestinal manifestations. Interestingly, the Annexin V+ compartment shows finer correlations to IBD status and might be implicated to both inflammatory and immune-mediated manifestations of IBD. Celiac disease represents a unique paradigm of disease characterized by gluten-induced enteropathy, in which removal of the trigger is generally followed by remission, as is demonstrated by the regression of atrophy after gluten exclusion. As such, it could be a useful model of disease/trigger interaction in EV research. Celiac disease is also associated with both autoimmune and immuno-mediated complications, whose development is frequently independent of the activity, severity or treatment response. Our data point toward a potential implication of EVs in systemic signaling, both at the initial and the later stages of CD. Further studies are needed to better understand the specific patterns, associations and effects of EVs and their potential role in CD-associated conditions of non-autoimmune origin.
