closed
Chiara Tortora, University “Luigi Vanvitelli”, Naples, Italy
Triennial Fellowship
Celiac Disease
Area: Clinics
Grant: FC 009/2017
- Title: The role of CB2 receptor in the pathogenesis of celiac disease and associated bone loss.
- Duration: Triennial Project
- Principal Investigator: Chiara Tortora, University Luigi Vanvitelli, Naples, Italy
- Tutor (Head Lab): Prof. Francesca Rossi, University “Luigi Vanvitelli”, Naples, Italy
Publications originating from the Project
- Tortora C, Punzo F, Argenziano M, Di Paola A, Tolone C, Strisciuglio C, Rossi F. The Role of Cannabinoid Receptor Type 2 in the Bone Loss Associated With Pediatric Celiac Disease. J Pediatr Gastroenterol Nutr. 2020 Nov;71(5):633-640. doi: 10.1097/MPG.0000000000002863. PMID: 33093370. https://pubmed.ncbi.nlm.nih.gov/33093370/
- Tortora C, Di Paola A, Argenziano M, Creoli M, Marrapodi MM, Cenni S, Tolone C, Rossi F, Strisciuglio C. Effects of CB2 Receptor Modulation on Macrophage Polarization in Pediatric Celiac Disease. Biomedicines. 2022 Apr 9;10(4):874. doi: 10.3390/biomedicines10040874. PMID: 35453624; PMCID: PMC9029516. https://pubmed.ncbi.nlm.nih.gov/35453624/
Project rationale and aims
Celiac disease (CD) is an autoimmune small intestinal disorder with a systemic and chronic inflammatory immune response against gluten in genetically predisposed individuals. Macrophages are key modulators of immune response and respond to a variety of environmental stimuli by acquiring either a pro-inflammatory (M1) or an anti-inflammatory (M2) phenotype. The contribution of macrophages to the pathogenesis of CD has been widely suggested. Cells of the immune system express the Cannabinoid Receptor type 2 (CB2). It is known that drugs acting on CB2 show an inhibitory effect on the immune system, causing a reduction of B and T lymphocyte proliferation; attenuation of phagocytosis and proinflammatory cytokine release in macrophages. In a previous study we demonstrated an association between the CB2 Q63R variant and CD, suggesting the receptor as a biomarker for the diagnosis of CD. Osteoporosis (OP) is a frequent extra-intestinal manifestation of CD. More than 50% of CD children show low bone mineral density (BMD) at the time of diagnosis. The ethology of OP in CD is multifactorial and mainly related to malabsorption of micronutrients and pro-inflammatory cytokines release that stimulates osteoclastogenesis. A lifelong gluten-free diet (GFD) is the only effective treatment for CD, nevertheless, it is not always enough to normalize BMD. CB2 is also involved in bone cell differentiation, survival and function and its stimulation reduces osteoclast activity in several bone pathologic conditions. For these reasons CB2 could be involved in the pathogenesis of CD and in associated bone loss. We evaluated CB2 expression and the phenotype of primary macrophages isolated from peripheral blood of CD patients, and the effects of the pharmacological modulation of CB2 in inducing CD macrophage polarization. Moreover, by in vitro model of “immunocompetent gut”, constituted by CD macrophages and the human epithelial cell line, Caco-2, we investigated the CB2 stimulation effects on intestinal barrier functionality evaluating inflammatory state and proliferation of caco-2 cells. We also investigated the expression of osteoclast biomarkers and CB2 in osteoclasts obtained from CD patients at diagnosis and after one year of GFD and evaluated the effects of the pharmacological modulation of CB2 on osteoclast activity.
Research plan and results obtained
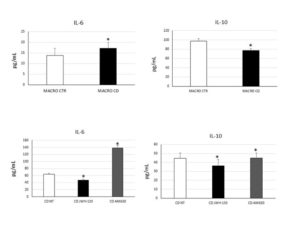
To achieve the first aim we evaluated CB2 and M1/M2 polarization markers (respectively CCR7/DMT1 and CD206/pSTAT6) expression. CB2 was significantly lower in CD-macrophages than CTR-macrophages and M1/M2 markers were respectively higher and lower compared with CTR-macrophages, suggesting that in CD there was a prevalence of the M1 phenotype. Moreover, we evaluated cytokine release by CD-macrophages confirming the existence of M1 phenotype. We treated in vitro CD macrophages with the CB2 agonist JWH-133 and the CB2 inverse agonist, AM630, analyzing the effects on M1/M2 markers expression and cytokine release. JWH-133 induced a polarization toward the anti-inflammatory M2 phenotype. AM630 induced opposite effects demonstrating the really involvement of CB2 in macrophage polarization. Then we co-cultured CD macrophages with Caco-2 cells. We treated this model of “immunocompetent gut with JWH-133 and AM630 and analyzed their effects on Caco-2 inflammatory state and viability. We observed a significant increase of IL-6 in Caco-2 co-cultured with CD macrophages untreated compared to Caco-2 alone. After JWH-133 administration we observed a reduction of the cytokine. AM630 increased IL-6 release by caco-2 worsening the basal condition. Moreover, we observed a reduction of viable Caco-2 when co-cultured with CD macrophages untreated compared to Caco-2 alone. These data suggest the contribute of CD M1 macrophages in inducing epithelium inflammation and damage. To achieve the second aim we evaluated the expression of osteoclast markers (TRAP/Cathepsin K) and CB2 in OCs derived from CD patients observing an increase of these markers, osteoclast number and size in CD patients. CB2 expression was lower in CD-OCs than CTR-OCs. We treated in vitro CD-OCs with JWH-133 and AM630 analysing the effects on OCs function and activity. JWH-133 induced a decrease of TRAP and Cathepsin K expression, while AM630 induced an increase of both markers. Colorimetric assays revealed a decrease of CD-OCs number and activity after JWH-133 administration and an increase after CB2 blockade with AM630. We also analyzed the effects of GFD on osteoclast activity evaluating TRAP and CB2 expression. Even if the basal condition have not been restored, OCs derived from CD patients on GFD, showed a reduction of TRAP and an increase of CB2 expression.
Experimental design and methodologies
For the first aim: 10 CD children at diagnosis and 10 healthy donors (CTR) were enrolled. Macrophages were isolated from peripheral blood mononuclear cells (PBMCs) which were cultured for 15 days in presence of rh-MCSF. For co-culture, Caco-2 were seeded into transwell inserts and maintained for 14/20 days. The insert with a fully differentiated Caco-2 monolayer was added into the transwell plate pre-loaded with CD macrophages. Mono/Co-cultures were treated with JWH-133 [100nM] and AM630 [10µM] for 48h. CB2, M1/M2 markers and cytokines expression were determined by western blotting (WB). Cytokine release was determined by ELISA assay; Caco-2 viability was determined by viability assay. For the second aim: 10 CD children at diagnosis, 10 CTR and 5 CD children after one year of GFD were enrolled. OCs were obtained from PBMCs which were cultured for 21 days in presence of rh-MCSF and RANK-L. OCs were treated with JWH-133 [100nM] and AM630 [10μM] for 48h. CB2, TRAP and Cathepsin K expression were determined by qPCR and WB. TRAP was evaluated by TRAP assay. OCs activity was evaluated by Bone Resorption assay. All the experiments were conducted in triplicate. Statistical analyse was performed using the non-parametric Wilcoxon test.
Potential pitfalls and caveats
Our project foresaw to study simultaneously macrophages isolated from the peripheral blood and from intestinal biopsies of celiac patients at diagnosis. Unfortunately, the low yield obtained for intestinal macrophages did not allow us to carry out the planned experiments.
Conclusions and discussion
Macrophages contribute to CD pathogenesis. It has been demonstrated that ex-vivo stimulation with gliadin induces pro-inflammatory activation not only of intestinal macrophages but also of circulating monocytes. In the intestinal lamina propria of CD patients, the macrophages derived from circulating monocytes could be recruited to the tissue and differentiated into M2 under the influence of the inflammatory microenvironment. We isolated macrophages from PBMC of CD patients and analyzed the possibility to induce a macrophage phenotype switch towards M2, by CB2 modulation. In CD-macrophages we found a reduction of CB2, an increase of M1 markers and pro-inflammatory cytokines release. After JWH-133 administration, we observed a phenotype switch toward the anti-inflammatory and immune suppressive M2 phenotype. We also evaluated the effects of CB2 modulation in in vitro model of “immunocompetent gut”, constituted by CD macrophages and Caco-2 cells, evaluating Caco-2 inflammatory state and viability. We observed an increase of IL-6 and a reduction of viable Caco-2 cells when CD macrophages and Caco-2 are co-cultured. JWH-133 inhibited the barrier damage induced by M1 CD macrophages as demonstrated by IL-6 reduction and by the restoring of caco-2 viability. Our data are in agreement with the well-known protective role of CB2 in inflammatory and immune regulation and suggest CB2 as a pharmacological target to revert the chronic inflammatory immune response of CD. OP has an high impact on CD patients health. More than 50% of CD children show low BMD at diagnosis. GFD is the only treatment for CD, however it is not sufficient to normalize BMD. We previously showed that CB2 stimulation inhibits bone resorption, demonstrating a protective role of the receptor in bone metabolism and suggesting it as a target for OP. We investigated osteoclast biomarkers and CB2 expression in OCs from CD patients at diagnosis and after one year of GFD. Moreover, we evaluated the effects of CB2 modulation on CD osteoclast activity and resorption. In CD patients we found an osteoclast hyper-activation and high levels of bone resorption markers and a reduction of CB2. JWH-133 reduced the osteoclastic hyper-activation, confirming CB2 as an inhibitor of bone resorption. We also investigated osteoclast biomarkers and CB2 expression in OCs obtained from CD after GFD for at least one year. We found a significant CB2 increase in CD patients on GFD. Nevertheless, the basal condition was not restored, suggesting the necessity of osteoprotective treatments in patients who have a persistent increased osteoclast activity after GFD. Our data suggest the necessity of an early diagnosis of the disease to counteract long-term complications related to bone health and highlight CB2 as a disease biomarker to identify CD patients at risk of bone alterations. Moreover, CB2 could be a pharmacological target to reduce bone mass loss in patients who need a direct intervention on bone metabolism, in addition to the GFD.
Figure 1. IL-6 and IL-10 concentrations (pg/mL) in CD macrophages (MACRO CD) compared with CTR macrophages (MACRO CTR), determined by enzyme-linked immunosorbent assay (ELISA) before and after treatment with JWH-133 [100nM] and AM630 [10μM] for 48h. Histograms show cytokines concentration as the mean ± S.D of independent experiments on each individual sample. The cytokines concentration was determined on a standard concentration curve according to the manufacturer’s instructions. The non-parametric Wilcoxon test was used for statistical analysis. * indicates p ≤ 0.05 compared to MACRO CTR and to CD untreated (NT).
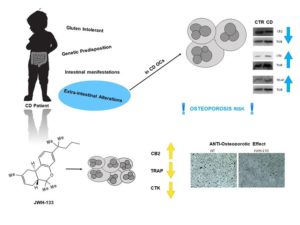
Figure 2. Celiac Disease (CD) predisposes to osteoporosis. Osteoclasts (OCs) from CD children have a reduced CB2 receptor expression and an osteoclast hyper-activation as demonstrated by the increased expression of OCs markers (CTK and TRAP). JWH-133, a CB2 agonist, reduces osteoclast activity.