closed
Federico Manai, University of Pavia, Italy
Triennial Fellowship
Celiac disease
Area: Oncology
Grant: FC 001/2018
- Title: Molecular and cellular effects of gluten exorphins on tumorigenesis: an in vitro pilot study.
- Duration: Triennial Project
- Principal Investigator: Federico Manai, University of Pavia, Italy
- Tutor (Head Lab): Prof. Sergio Comincini, University of Pavia, Italy
Publications originating from the Project
- Manai F, Zanoletti L, Morra G, Mansoor S, Carriero F, Bozzola E, Muscianisi S, Comincini S. Gluten Exorphins Promote Cell Proliferation through the Activation of Mitogenic and Pro-Survival Pathways. Int J Mol Sci. 2023 Feb 15;24(4):3912. doi: 10.3390/ijms24043912. PMID: 36835317; PMCID: PMC9966116. https://pubmed.ncbi.nlm.nih.gov/36835317/
- Zanoletti L, Valdata A, Nehlsen K, Faris P, Casali C, Cacciatore R, Sbarsi I, Carriero F, Arfini D, van Baarle L, De Simone V, Barbieri G, Raimondi E, May T, Moccia F, Bozzola M, Matteoli G, Comincini S, Manai F. Cytological, molecular, cytogenetic, and physiological characterization of a novel immortalized human enteric glial cell line. Front Cell Neurosci. 2023 Apr 20;17:1170309. doi: 10.3389/fncel.2023.1170309. PMID: 37153631; PMCID: PMC10158601. https://pubmed.ncbi.nlm.nih.gov/37153631/
THE STUDY
Project rationale and aims
Gluten exorphins (GEs) are small biologically active peptides released from the gastrointestinal enzymatic digestion of gluten. It has been demonstrated that these peptides can cross the intestinal epithelial barrier (IEB), enter the blood stream, and bind the opioid receptors, particularly the δ-opioid receptor (DOR), thus leading to their activation. Recently, the opioid effect of GEs has been proposed as a main protagonist in the onset of asymptomatic celiac disease (CD), a form of this disorder in which the subjects do not experience any symptom even in presence of intestinal damage (i.e., villous atrophy, crypt hyperplasia, and lymphocytes infiltration). There is emerging evidence that opioid-mediated signalling plays a role in cancer progression by interacting with MAPK, ERK and other signalling pathways, controlling cell proliferation and apoptosis. As already known, untreated CD is associated with enterophaty-associated T-cell lymphoma (EATL) and small bowel carcinoma (SBC). Considering these premises as well as the absence of data regarding the role of GEs in CD, this study investigated the effect of GEs in in vitro cellular models of lymphocytes and enterocytes, based on SUP-T1 and Caco-2 cells. Moreover, GEs were tested also in an in vitro model of human immortalized enteric glial cells (EGCs) generated in our laboratory through a validated lentiviral transduction protocol. EGCs, indeed, together with enteric neurons, play an important role in intestinal homeostasis in both physiological and pathological conditions. Although their role in Crohn disease and ulcerative colitis is known, no evidence regarding CD are present in literature. The aim of the Project was to describe the effects of GEs also in EGCs to identify new cellular/molecular mechanisms involved in CD onset.
Research plan and results obtained
The first part of the research plan of the Project was aimed at verify the effect of GEs in an in vitro model T-cells, based on SUP-T1 cells. Specifically, synthetic GEs were initially tested at different concentrations in SUP-T1 cells investigating their role in increasing cell viability/proliferation and cell cycle progression. GEs promoted an increase in proliferation and cell cycle progression starting from the concentrations reported in the BIOPEP database. Moreover, no cytotoxic effects were detected increasing their concentration from micromolar to millimolar. Conversely, no effects were observed on human primary fibroblasts. According to MTS assay, novel experimental useful concentrations were identified and investigated in the subsequent experiments. After four hours of GEs treatment, SUP-T1 cells showed an increase in the phosphorylation of downstream opioid receptor kinases (i.e., Akt and Erk1/2) as well as in anti-apoptotic and pro-survival factors (i.e., Bcl-2 and LC3-II). Exorphin B5 was confirmed to be the most potent one, as already described in literature. This exorphin was then selected and tested at increasing concentration in Caco-2 cells. Again, an increase in cell metabolism was observed at four hours post-treatment with the highest concentration tested, together with an increase in Erk1/2 phosphorylation. Conversely, at the same concentration range, a reduction in LC3-II and STAT3 phosphorylation was observed.
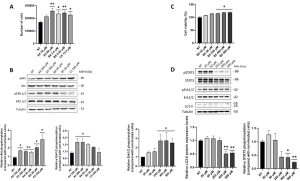
Figure 1. Analysis of cell viability/proliferation and relative pathways in SUP-T1 and Caco-2 cells following GEs administration four hours post-treatment. (A) Proliferation assay of SUP-T1 cells by means of flow cytometry analyses using the GEs concentrations reported in the BIOPEP database (i.e., A4 = 70 μM; A5 = 60 μM; B4 = 3.4 μM; B5 = 0.02 μM; C5 = 13.5 μM). The assay was performed 24 hours post-treatment. (B) Immunoblotting and relative densitometric analysis of SUP-T1 treated with GEs at the concentrations (i.e., A4 = 350 μM; A5 = 300 μM; B4 = 50 μM; B5 = 20 μM; C5 = 250 μM) determined with MTS assays (data not shown). Akt and Erk1/2 activation levels were investigated four hours post-treatment. (C) MTS assay of Caco-2 cells treated with increasing concentrations of B5 exorphin (i.e., 20, 50, 250, 400, and 500 μM) at four hours post-treatment. (D) Immunoblotting and relative densitometric analysis of Caco-2 treated with B5 exorphin at increasing concentrations (i.e., A4 = 350 μM; A5 = 300 μM; B4 = 50 μM; B5 = 20 μM; C5 = 250 μM). LC3-II, STAT3 and Erk1/2 activation levels were investigated four hours post-treatment. Normalized values are reported on Y-axis as arbitrary units. Molecular weights (MW) in kDa and SEM bars are reported. Asterisks indicate p<0.05 (*) or p<0.01 (**) compared with non-treated (NT) control cells. The experiments were performed on at least three independent biological replicas.
Exorphin B5 was also tested at increasing concentrations in immortalized EGCs. Starting from one hour post-treatment, an increase in cell metabolism was observed. An mRNAseq analysis was then performed to identify alterations in gene expression. A down-regulation was observed for genes implicated in cell cycle progression whereas an up-regulation was detected for EDN1 gene, which encoded for endothelin-1, a protein highly increased in gut inflammation.
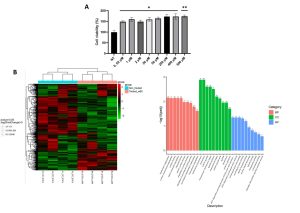
Figure 2. Analysis of B5 exorphin effects on immortalized human EGCs. (A) MTS assay of ClK treated with B5 exorphin at increasing concentrations one hour post-treatment. (B) On the left, heatmap reporting the DGEA of ClK cells treated with B5 exorphin one hour post-treatment. The threshold was set at log2FoldChange. Clustering was performed using the log2(FPKM+1) value. Red colour indicates up-regulated genes whereas green down-regulated genes. On the right, GO enrichment analysis histogram. The abscissa in GO Term, and the ordinate is GO Term’s levels of significance of enrichment, expressed as -log10(padj). Different colours represent different functional categories: in pink Biological Processes, in green Cellular Component, and in blue Molecular Function.
Experimental design and methodologies
The proliferation assays were performed by counting cells through flow cytometry whereas the viability/metabolic activity was measured using the MTS assay. The analysis of the cell cycle in SUP-T1 cells was carried out synchronizing them with colcemid, a derivative of colchicine capable of arresting cells in metaphase, in order to further confirm the proliferative effects of GEs. The cell cycle phases were studied by flow cytometry using propidium iodide to stain the DNA. Immunoblotting was then used to investigate the pathways of interest, particularly by analyzing the levels of the target protein and their post-translational modifications (i.e., phosphorylation). The mRNAseq analysis was performed to unveil GEs effects on EGCs, since no hypothesis regarding possible beneficial or detrimental responses as well as candidate pathways were present in literature.
Potential pitfalls and caveats
The major pitfall was represented by the Sars-CoV-2 pandemic, which prevented the obtaining of intestinal biopsies from CD patients and controls to derive primary EGCs. As a solution to this problem, an immortalized human EGC line (ClK) was generated in our laboratory using a validated lentiviral transduction protocol.
Conclusions and discussion
To date, the effect of GEs released from the digestion of gluten was not deeply investigated. However, it has been demonstrated that GEs exert different effects in several physiological contests, also depending on their ability to cross the intestinal epithelial barrier. The obtained results demonstrated that GEs stimulate the proliferation in SUP-T1 cells as also corroborated by a cell cycle analysis on synchronized cells. The increase in cell proliferation relies on the phosphorylation of Akt and Erk1/2, as demonstrated by immunoblotting. Moreover, the proliferative effect of GEs was accompanied by an increase in Bcl-2, ant anti-apoptotic protein triggered by IL-15 in IELs, and in the autophagy marker LC3-II. B5 exorphin was then selected and investigated in Caco-2 cells. This exorphin promoted an increase in cell metabolism and in Erk1/2 phosphorylation. Moreover, the reduction in LC3-II and phospho-STAT3 levels suggested that GEs can trigger a loss of homeostasis in intestinal epithelial cells, considering the beneficial role of these two proteins in IECs. The results collected in Caco-2 cells lead to the hypothesis that GEs could contribute to the detrimental effects exerted by gliadin on IECs. This hypothesis is also strengthened by the effects exerted by B5 exorphin in ClK cells. The mRNAseq analysis performed on this EGC line revealed that B5 led to the down-regulation of gene involved in cell cycle regulation and in increase in EDN1 expression, a gene which encoded for endothelin-1, a protein highly increased during gut inflammation that contribute to trigger autocrine and paracrine pathways since EGCs and other cells express its receptor. Altogether, these results suggested a possible role of GEs in CD pathogenesis. Particularly, GEs could contribute to induce lymphocytes activation and infiltration as well as promoting IECs loss of homeostasis and EGCs activation, thus promoting inflammation.
